21+ Calculate The Mass Of The Reaction Mixture In The Calorimeter
Pages 550 words Approximate price. Dissolve 1 mol of NaCl and 1 mol of KNO3 in a liter of water.
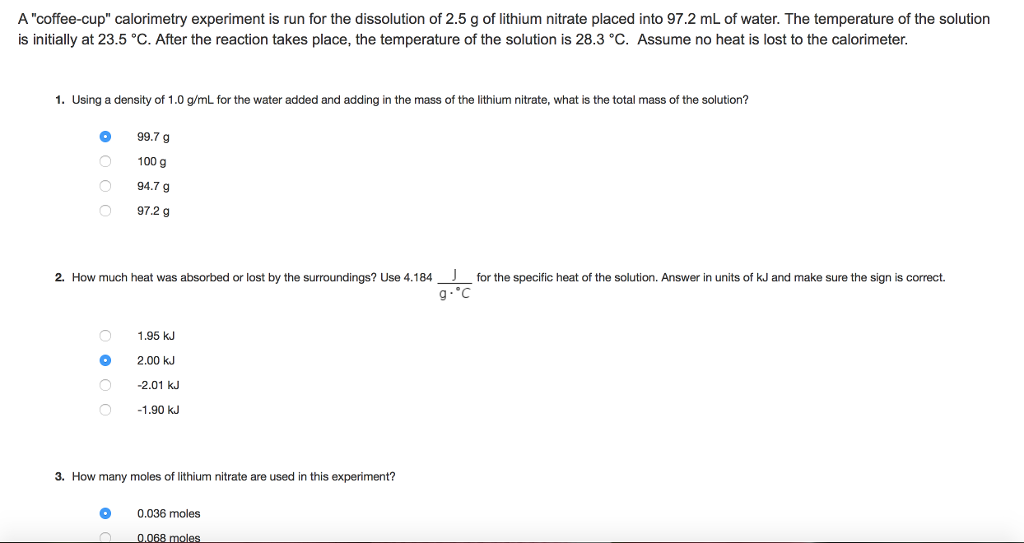
Solved If The Lid Was Left Off During The Temperature Chegg Com
The final temperature of the reaction mixture is recorded as 2277C.
. Web When 321 g of solid NH 4 NO 3 dissolves in 500 g of water at 249 C in a calorimeter the temperature decreases to 203 C. What is the mole fraction of O2 in a mixture that is 790 mole N2 and 210 mole O2. Calculate the volume of capillary pores.
Web A group of Chem 161 students was asked to determine the heat of reaction forthe neutralization of a newly synthesized monoprotic acid HX and NaOH. Web This more accurately reflects the species that are found in the reaction mixture. Thereafter the skin of the neck was shaved and sterilized with 70.
First we need to calculate the heat of reaction using formula Q mCT Then we need to calculate. This is very nicely demonstrated when two different salts are dissolved in water. How much heat did the water absorb.
Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. G of water is heated and the temperature of the water increases from 21 C to 85 C. Web Enthalpy ˈ ɛ n θ əl p i a property of a thermodynamic system is the sum of the systems internal energy and the product of its pressure and volume.
613 g O21 mol O231998 g O26 mol H2O5 mol O2180148 g H2O1 mol H2O414 g H2O. What is the energy value kcalg for the oil. Determine the enthalpy of reaction for the neutralization reaction expressed in kJ per mol H 2 O formed.
I Calculate the magnitude of the heat energy in joules that is released during the reaction. Web Mice were anaesthetized by an intraperitoneal injection of ketamine 100 mg kg 1 and xylazine 10 mg kg 1 mixture. 19 Each energy source carbohydrate protein and fat is oxidized at a known RQ ranging from 07 to 1 Table 199-1Therefore the RQ can be.
Web When 500 mL of 0100 M AgNO3 is combined with 500 mL of 0100 M HCl in a coffee-cup calorimeter the temperature changes from 2340 C to 2421 C. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. Web Initially the temperature of the reaction mixture in the calorimeter styrofoam cup increases as HCl aq is added.
Web the addition of 100g of a compound at 750 g of CCL4 caused a cryoscope lowering of 105 K. What is the approximate amount of heat produced by this reaction. Web Next calculate the amount of H2O that can be produced from 613 g O2 and excess NH3.
State any assumptions that you made. You fill in the order form with your basic requirements for a paper. Web 500 mL of 20 M HCl and 500 mL of 20 M NaOH both initially at 211 C are added to a calorimeter and allowed to react.
Web A 0800-g piece of Zn metal is added to an insulated coffee-cup calorimeter containing 500 g of an aqueous AgNO₂ solution at a temperature of 1925C. Use 100 gmL as the density of the solution and C 418 Jg C as the specific heat capacity. A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume.
Calculate Hrxn for the reaction as written. The temperature rises to 315 C. Theyfirst calibrated the styro ball calorimeter using the reaction of 150 mL of 0100 MHCl and 200 mL of 00500 M KOH.
AgaqClaqAgCls The two solutions were initially at 2260C and the final temperature is 2340C Calculate the heat that accompanies this reaction in kJmol of AgCl formed. Assume 100 hydration and no drying. Energy heat is being produced by the reaction.
In a constant-pressure calorimeter 550 mL of 0310 M BaOH₂ was added to 550 mL of 0620 M HCl A. When the sample is burned 189 kJ is given off. Calculate the molar mass of the compound.
Web Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed. Web Study with Quizlet and memorize flashcards containing terms like which of the following correctly reflect Avogadros number. Web Calculate your essay price.
Web In a coffee-cup calorimeter 1500 mL of 050 M HCI is added to 500 mL of 100 M NaOH to make 2000 g solution at an initial temperature of 482C. Hence V cem 100315 318 ml M water 50 g therefore V w. The titrant reacts with a solution of analyte.
Web Our custom writing service is a reliable solution on your academic journey that will always help you if your deadline is too tight. If the enthalpy of neutralization for the reaction between a strong acid and a strong base is 56 kJmol calculate the final temperature of the calorimeter contents. Web In a coffee-cup calorimeter 500 mL of 0100 M AgNO3 and 500 mL of 0100 M HCl are mixed to yield the following reaction.
Containing a stirred reaction mixture beaker wide cylinder shape - pouring an approximate volume of liquid into a buret. Measurements using a conduction calorimeter can give the rates of heat evolution at various stages. Web A calorimeter measures the ___ involved in reactions or other processes by measuring the ___of the materials ___ the process.
This recorded a change in temperature from265 C to 318 C. The heat capacity of the calorimeter is 25 JC. - the mole contains 6022 x 1023 entities - for a pure substance the mole specifies only the number of entities in a sample - the number of entities in a mole is called Avogadros number - the mole is abbreviated mo what correctly.
The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Your academic level paper type and format the number of pages and sources discipline and deadline. In fact KNO3 is actually two different compounds entities in a single crystal lattice.
Select the correct calculations needed to find H for the reaction shown below in kJmol Zn. For O2 the molar mass is 215999 gmol31998 gmol and there are six mol H2O produced per five mol O2that react. This lets us find the most appropriate writer for any type of assignment.
Web The reaction of cement hydration is exothermic. Conservation of Mass Counting. Maximum temperature reached is 246C when 500 mL of HCl aq had been added.
Assume that the specific heat of the reaction mixture is 394. Web Calculate the heat lost or gained by measuring its mass and temperature change. These ions are hydrated.
Web An additional feature of the metabolic cart is the ability to calculate not only the REE but the respiratory quotient RQ from the measured andThe RQ is the ratio of and it can be used as an indicator of substrate use. The reaction is exothermic. Web A flask containing 80 times 102.
Calculate the mass percent of a vinegar solution with a total mass of 9641 g that. If an industrial hydrogenator with a volume of 500 litre is charged with 24 kilograms of oil and the. Web e The mass of the reaction mixture inside the calorimeter is 1521 g.
Atoms are never created or destroyed during a chemical reaction. When 500 mL of the acid has been added all the base has been. Let mass of cement 100 g.
Web Heat Produced by an Exothermic Reaction When 500 mL of 100 M HClaq and 500 mL of 100 M NaOHaq both at 220 C are added to a coffee cup calorimeter the temperature of the mixture reaches a maximum of 289 C. A 050-g sample of vegetable oil is placed in a calorimeter. It is a state function used in many measurements in chemical biological and physical systems at a constant pressure which is conveniently provided by the large ambient atmosphere.

Calorimetry Chemistry Socratic
Browse Questions For Chemistry 101

Solved Table 1 3 Mass Of Calorimeter Mass Of Calorimeter Chegg Com
Solved Part Ii Calculate The Energy During A Chemical Chegg Com
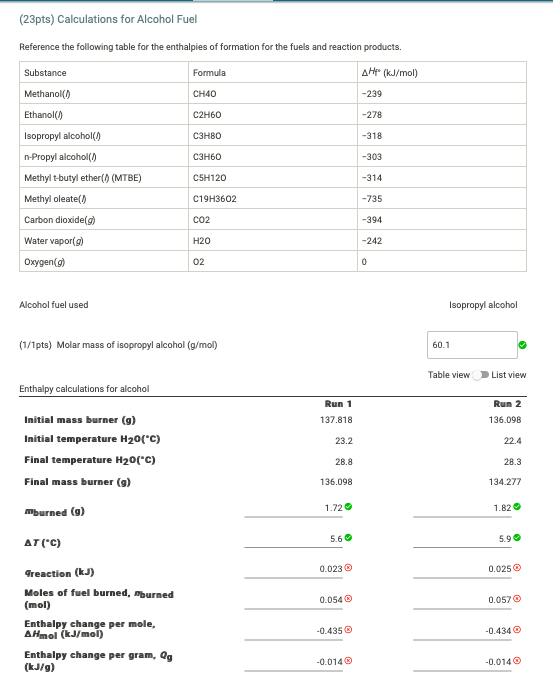
Solved Table View List View Table 3 Calculations For Chegg Com

Solved In Parts 2 3 And Of This Lab You Will Be Conducting Calorimetry Experiments Two Solutions Each Of Volume 2 5 Ml Will Neutralization Reactions In Solution Be Mixed Together
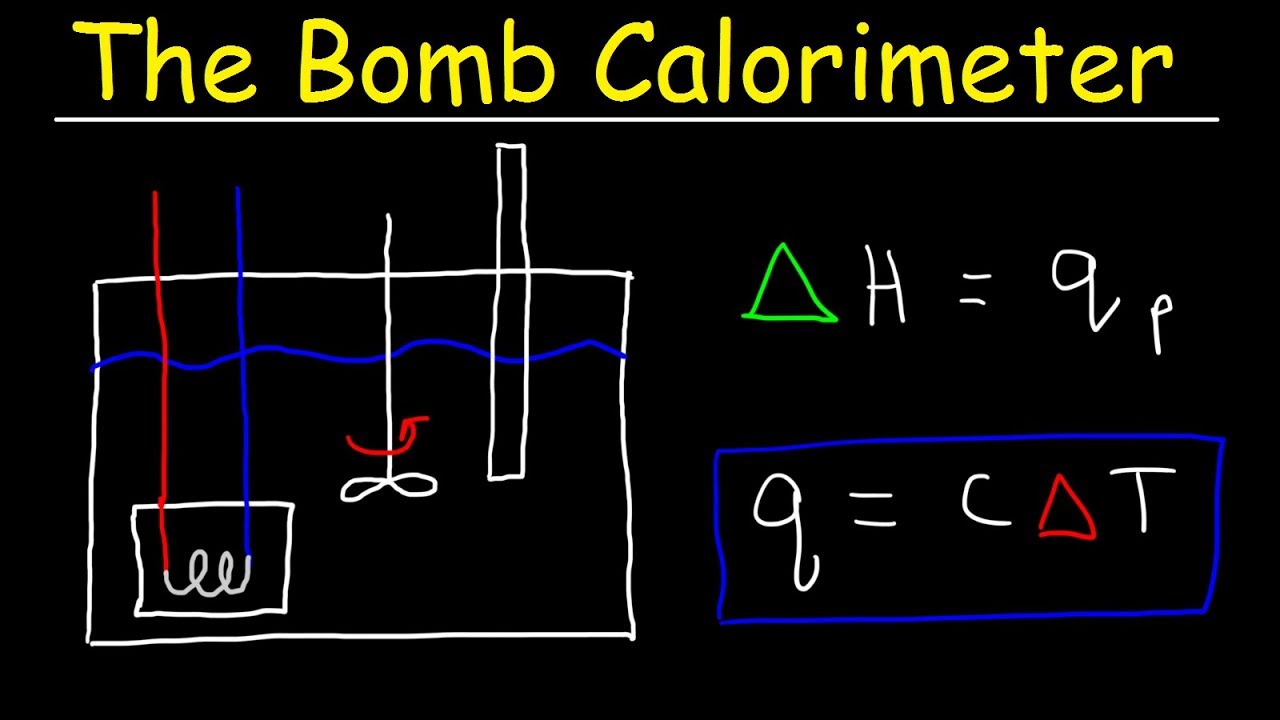
Coffee Cup Calorimeter Calculate Enthalpy Change Constant Pressure Calorimetry Youtube

Solved Data And Calculations Mass Of Clean Dry Calorimeter Chegg Com

How To Calculate The Heat Gained By The Calorimeter Sciencing
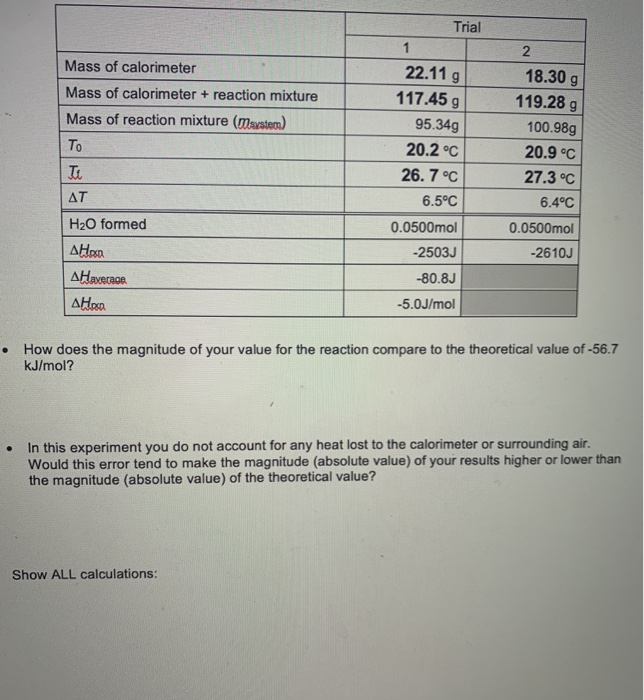
Solved Mass Of Calorimeter Mass Of Calorimeter Reaction Chegg Com
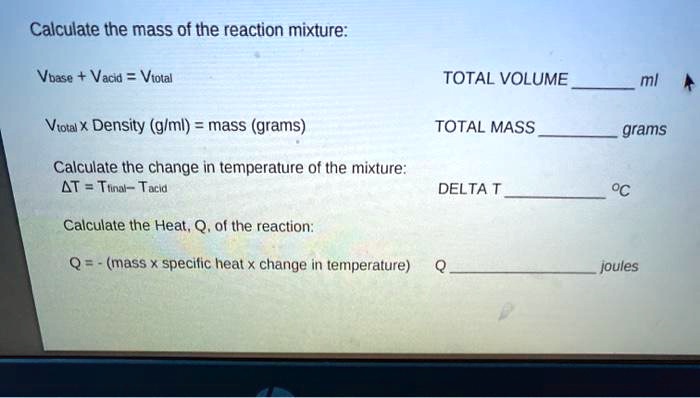
Solved Calculate The Mass Of The Reaction Mixture Vbase Vacid Violal Total Volume Mi Viotal Density Glml Mass Grams Total Mass Grams Calculate The Change In Temperature Of The
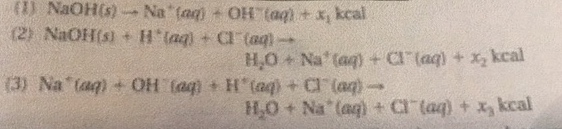
Solved Calculate The Mass Of The Reaction Mixture In Each Chegg Com

Pdf Coffee Cup Calorimeter Heat Loss Correction
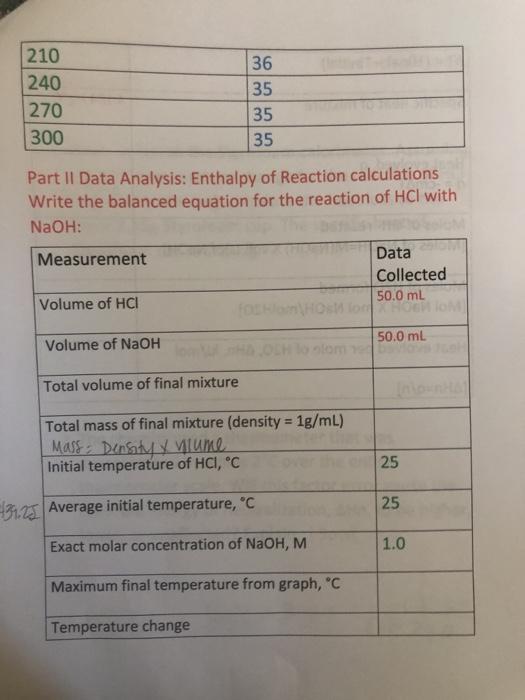
Solved Data Sheet Calorimetry Table 1 Calorimeter Chegg Com

5 6 Calorimetry Chemistry Libretexts

Measuring Dhrxn In A Coffee Cup Calorimeter Chemistry Youtube
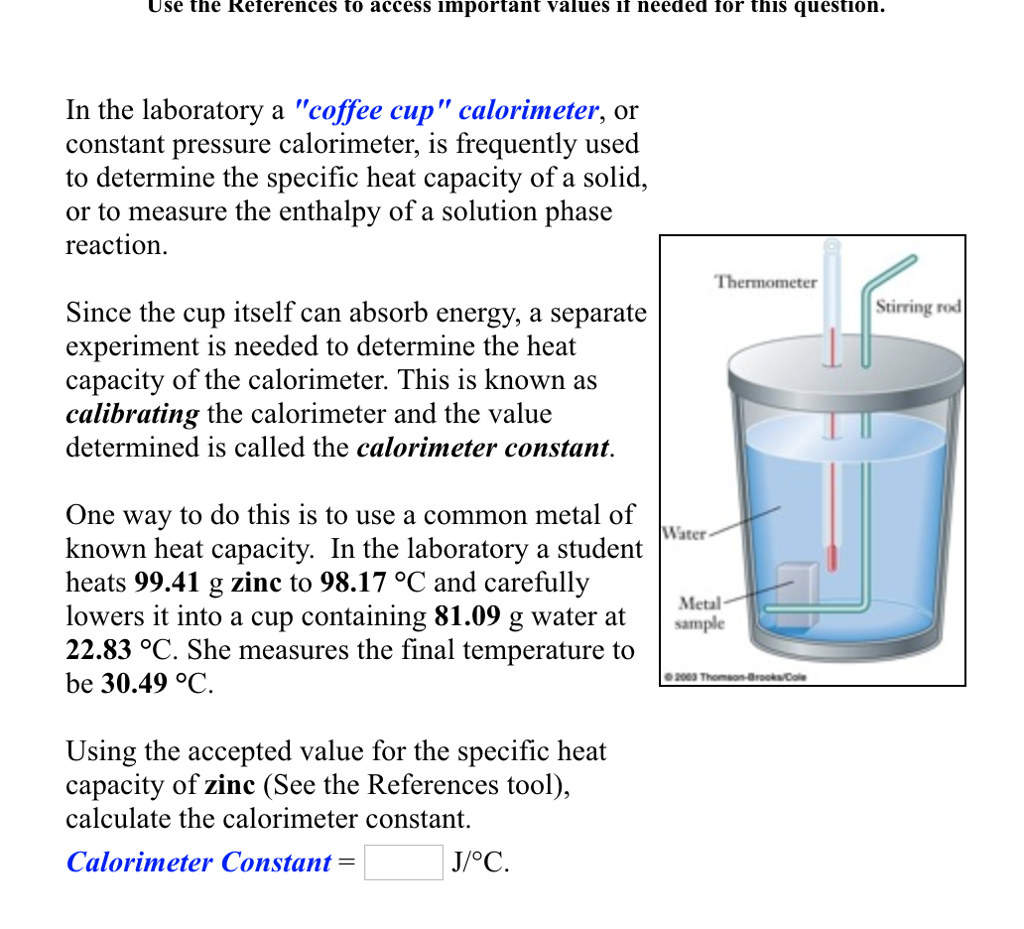
Solved Use The References To Access Important Values If Chegg Com